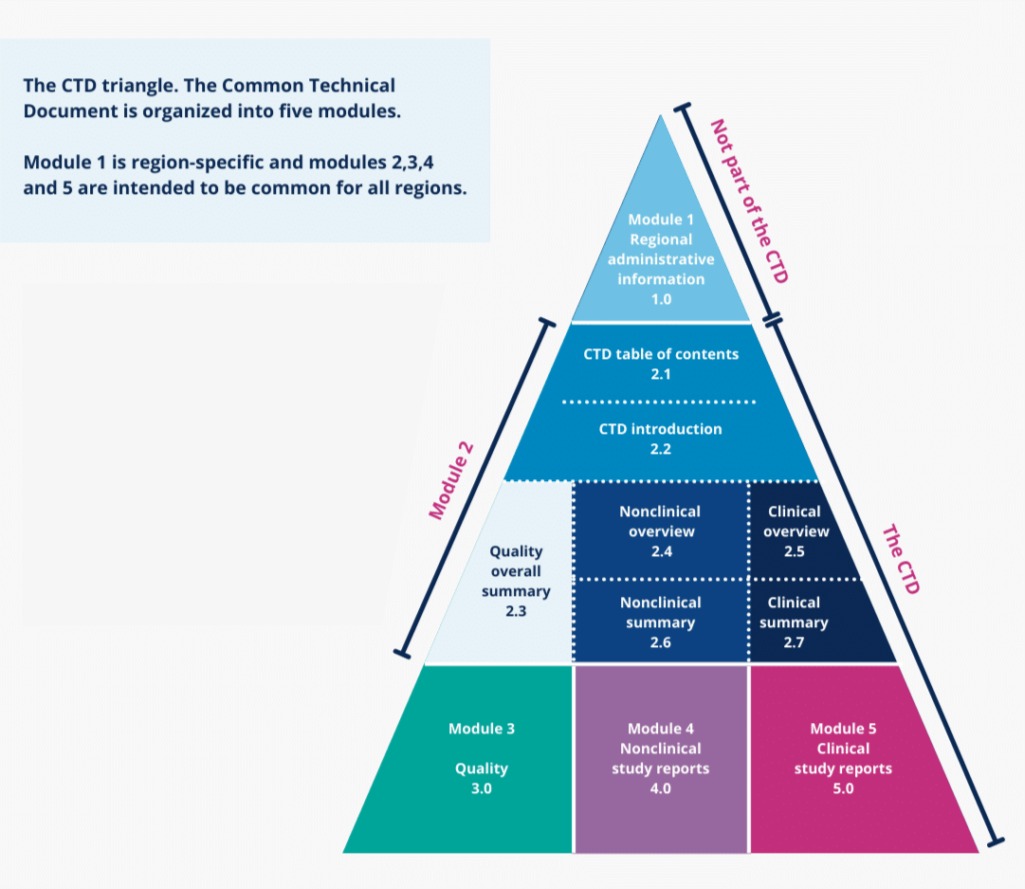
We specialize in preparing CTD/ACTD dossiers as per local & international requirements to facilitate our clients in timely submissions to regulatory authorities by assuring comprehensive documentation. We believe in continuous learning to deliver up-to-date guidance to clients for smooth processes.
Contact Us
INSTITUTE OF BIOLOGICAL, BIOCHEMICAL & PHARMACEUTICAL SCIENCES (IBBPS)
